A solid performance in the classroom study

The EVEKEO classroom study included 97 children with ADHD1
Before we tell you the classroom study results, you may be wondering what a classroom study is. Here’s how the researchers explain it:

A laboratory classroom setting for study of ADHD medications allows for assessment by trained observers over the course of a typical extended school day….1*

How pediatric study patients with ADHD were evaluated
In a classroom study of children ages 6 to 12 years with ADHD, the researchers were looking to see how EVEKEO® CII (amphetamine sulfate tablets, USP) affected the following1:
The children were evaluated multiple times over a 10-hour span on 2 different classroom days. On one classroom day, they received EVEKEO. On the other classroom day, they received a pill that looks exactly like EVEKEO but doesn't contain any medication, called a placebo. Results from the 2 days were compared to determine not only the effect EVEKEO had on symptoms, but also how long the effect lasted.1
Children who were excluded from the study included those with a primary psychiatric diagnosis other than ADHD, or those with a secondary or co-morbid diagnosis other than ADHD with the exception of simple phobias, oppositional defiance disorders, motor skill disorders, communication disorders, learning disorders, adjustment disorders, and sleep disorders. Others who were excluded from the study were children with clinically significant cognitive impairment or a personal history of seizure disorders (except simple febrile seizures), untreated thyroid disease, glaucoma, Gilles de la Tourette's disorder, or chronic tics, as well as those with a personal history of cardiac disorders, serious cardiac conditions, or severe hypertension.1
PERMP Test Results From a Real Classroom Study Subject With ADHD2
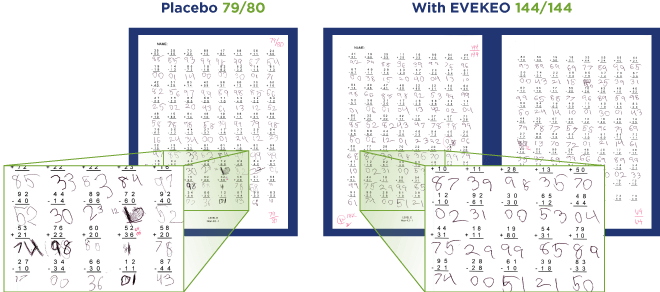
The results
EVEKEO significantly improved performance, behavior, and attention at every measured time point, from 45 minutes to 10 hours.1
- Improved performance: Children attempted to solve more problems on the PERMP, and answered more problems correctly, after taking EVEKEO compared with placebo (see example below)
- Improved behavior and attention: When the scores of the 13-item scale were combined, EVEKEO was shown to have significantly improved behavior and attention measured by the SKAMP scale compared with placebo
References: 1. Childress AC, Brams M, Cutler AJ, et al. The efficacy and safety of Evekeo, racemic amphetamine sulfate, for treatment of attention-deficit/hyperactivity disorder symptoms: a multicenter, dose-optimized, double-blind, randomized, placebo-controlled crossover laboratory classroom study. J Child Adolesc Psychopharmacol. 2015;25(5):402-414. 2. Data on file. Arbor Pharmaceuticals, LLC.
IMPORTANT SAFETY INFORMATION
Evekeo® (amphetamine sulfate tablets, USP) is a central nervous system (CNS) stimulant prescription medicine used for the treatment of:
- A sleep disorder called narcolepsy.
- Attention-Deficit Hyperactivity Disorder (ADHD) in children 3 to 16 years of age. Evekeo may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD.
- Exogenous obesity. Evekeo may be used as part of a short-term (a few weeks) weight reduction program for obesity in people who have not responded to other treatment.
IMPORTANT SAFETY INFORMATION
Evekeo® (amphetamine sulfate tablets, USP) is a central nervous system (CNS) stimulant prescription medicine used for the treatment of:
- A sleep disorder called narcolepsy.
- Attention-Deficit Hyperactivity Disorder (ADHD) in children 3 to 16 years of age. Evekeo may help increase attention and decrease impulsiveness and hyperactivity in people with ADHD.
- Exogenous obesity. Evekeo may be used as part of a short-term (a few weeks) weight reduction program for obesity in people who have not responded to other treatment.
Evekeo is not for use in children under 3 years of age.
It is not known if Evekeo is safe and effective in children with exogenous obesity under 12 years of age.
Evekeo is a federally controlled substance (CII) because it contains amphetamine that can be a target for people who abuse prescription medicines or street drugs. Keep Evekeo in a safe place to protect it from theft. Never give your Evekeo to anyone else because it may cause death or harm them. Selling or giving away Evekeo may harm others and is against the law.
WARNING: ABUSE, MISUSE, AND ADDICTION
Amphetamine sulfate has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including amphetamine sulfate, can result in overdose and death, and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing amphetamine sulfate, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout amphetamine sulfate treatment, reassess each patient’s risk of abuse, misuse, and addiction and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
ADDITIONAL IMPORTANT SAFETY INFORMATION
Do not take Evekeo if you or your child:
- are allergic to amphetamine products or any of the ingredients in Evekeo.
- are taking or have taken within the past 14 days, a medicine used to treat depression called a monoamine oxidase inhibitor (MAOI).
What is the most important information I should know about Evekeo?
Evekeo may cause serious sides effects, including:
Abuse, misuse, and addiction. Evekeo has a high potential for abuse and misuse and may lead to substance use problems, including addiction. Misuse and abuse of Evekeo, other amphetamine-containing medicines, and methylphenidate, can lead to overdose and death. The risk of overdose and death is increased with higher doses of Evekeo or when it is used in ways not approved, such as snorting or injection.
- Your healthcare provider should check you or your child’s risk for abuse, misuse, and addiction before starting treatment with Evekeo and will monitor you or your child during treatment.
- Evekeo may lead to physical dependence after prolonged use, even if taken as directed by your healthcare provider.
- Do not give Evekeo to anyone else.
- Tell your healthcare provider if you or your child have ever abused or been dependent on alcohol, prescription medicines, or street drugs.
Risks for people with serious heart problems. Sudden death has happened in people who have heart defects or other serious heart disease.
Your healthcare provider should check you or your child carefully for heart problems before starting treatment with Evekeo. Tell your healthcare provider if you or your child have any heart problems, heart disease, or heart defects.
Call your healthcare provider right away or go to the nearest hospital emergency room right away if you or your child have any signs of heart problems such as chest pain, shortness of breath, or fainting during treatment with Evekeo.
Increased blood pressure and heart rate. Your healthcare provider should check you or your child’s blood pressure and heart rate regularly during treatment with Evekeo.
Mental (psychiatric) problems can occur including:
- new or worsening behavior and thought problems.
- new or worsening bipolar illness.
- new psychotic symptoms (such as hearing voices, or seeing, or believing things that are not real) or new manic symptoms.
Tell your healthcare provider about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your healthcare provider right away if you or your child have any new or worsening mental symptoms or problems during treatment with Evekeo, especially hearing voices, seeing, or believing things that are not real, or new manic symptoms.
Before taking Evekeo, tell your healthcare provider about all of your or your child’s medical conditions, including if you or your child:
- have heart problems, heart disease, heart defects, or high blood pressure.
- have mental problems including psychosis, mania, bipolar illness, or depression, or have a family history of suicide, bipolar illness, or depression.
- have seizures or have had an abnormal brain wave test (EEG).
- have circulation problems in fingers and toes.
- have or had repeated movements or sounds (tics) or Tourette’s syndrome or have a family history of tics or Tourette’s syndrome.
- are pregnant or plan to become pregnant. It is not known if Evekeo will harm the unborn baby. Tell your healthcare provider if you or your child become pregnant during treatment with Evekeo.
- are breastfeeding or plan to breastfeed. Evekeo passes into breast milk. You or your child should not breastfeed during treatment with Evekeo. Talk to your healthcare provider about the best way to feed the baby during treatment with Evekeo.
Tell your healthcare provider about all of the medicines that you or your child take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Evekeo and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be changed during treatment with Evekeo. Your healthcare provider will decide if Evekeo can be taken with other medicines.
- selective serotonin reuptake inhibitors (SSRIs)
- medicines used to treat migraine headaches called triptans
- lithium
- tramadol
- buspirone
- serotonin norepinephrine reuptake inhibitors (SNRIs)
- tricyclic antidepressants
- fentanyl
- tryptophan
- St. John’s Wort
Know the medicines that you or your child take. Keep a list of your or your child’s medicines with you to show your healthcare provider and pharmacist when you or your child get a new medicine. Do not start any new medicine during treatment with Evekeo without talking to your healthcare provider first.
What should I avoid while taking Evekeo?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how Evekeo affects you.
What are possible side effects of Evekeo?
Evekeo may cause serious side effects, including:
- Slowing of growth (height and weight) in children. Children should have their height and weight checked often during treatment with Evekeo. Your healthcare provider may stop your child’s Evekeo treatment if they are not growing or gaining weight as expected.
- Seizures. Your healthcare provider may stop treatment with Evekeo if you or your child have a seizure.
- Circulation problems in fingers and toes (peripheral vasculopathy, including Raynaud’s phenomenon). Signs and symptoms may include:
- fingers or toes may feel numb, cool, painful.
- fingers or toes may change color from pale, to blue, to red.
Tell your healthcare provider if you or your child have numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your healthcare provider right away if you or your child have any signs of unexplained wounds appearing on fingers or toes during treatment with Evekeo.
- Serotonin syndrome. This problem may happen when Evekeo is taken with certain other medicines and may be life-threatening. Call your healthcare provider or go to the nearest hospital emergency room right away if you or your child develop any of the following signs and symptoms of serotonin syndrome:
- agitation, hallucinations, coma
- fast heartbeat
- flushing
- seizures
- sweating or fever
- loss of coordination
- confusion
- dizziness
- muscle stiffness or tightness
- changes in blood pressure
- high body temperature (hypothermia)
- nausea, vomiting, diarrhea
- New or worsening tics or worsening Tourette’s syndrome. Tell your healthcare provider if you or your child get any new or worsening tics or worsening Tourette’s syndrome during treatment with Evekeo.
The most common side effects of Evekeo include:
- headache
- stomachache
- trouble sleeping
- decreased appetite
- unpleasant taste
- nervousness
- dizziness
- sexual problems (impotence in males)
- vomiting
- itching
- diarrhea or constipation
- dry mouth
- weight loss
- mood swing
These are not all the possible side effects of Evekeo. Please see Full Prescribing Information for a full list.
The Important Safety Information does not include all the information needed to use Evekeo safely and effectively. Please see accompanying full Prescribing Information for Evekeo.
To Report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
PP-EVE-US-0547